About Us
Our mission is to provide best-in-class solutions for emerging neuromodulation and neurodiagnostic applications.


Neurotechnology for Advanced Medical Applications
IRIS Biomedical is a wholly owned subsidiary of Ripple Ventures established to provide neurotechnology engineering, and manufacturing services and technology licensing to the medical device industry.

Company Info
IRIS Biomedical is a subsidiary of Ripple Neurotechnology Ventures.
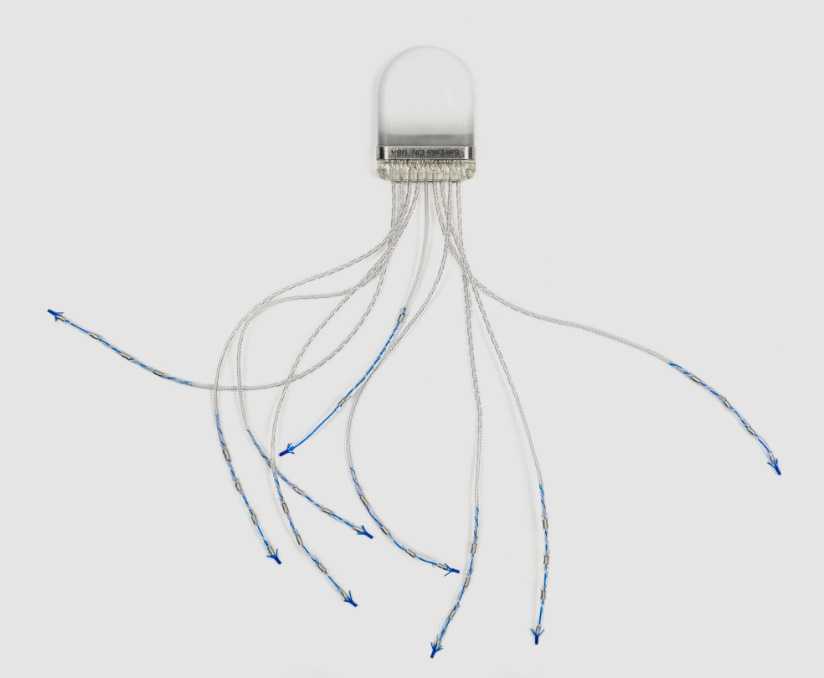
As an original design manufacturer (ODM) IRIS has designed its core technology platform from the ground up to offer a range of sub-component and complete-system designs. IRIS specializes in high-performance closed-loop neural sensing and stimulation. We also offer a range of components for continuous wireless power delivery, inductive battery charging, data telemetry, and portable signal processing. Our designs have been rigorously field tested and can be easily adapted to a variety of form factors.
IRIS is an ideal technology partner for early-stage medical device companies without the budget or expertise to invest in developing a new device from the ground up. Our experienced engineering team and in-house clean-room facilities allow for rapid design development as well as prototype and early-stage commercial production.
IRIS helps companies develop best-in-class products and get to market first. IRIS follows national and international medical device regulations. All design and manufacturing work is performed under an ISO:13485-certified Quality Management System.
Company Info

IRIS Biomedical is a subsidiary of Ripple Neurotechnology Ventures.
As an original design manufacturer (ODM) IRIS has designed its core technology platform from the ground up to offer a range of sub-component and complete-system designs. IRIS specializes in high-performance closed-loop neural sensing and stimulation. We also offer a range of components for continuous wireless power delivery, inductive battery charging, data telemetry, and portable signal processing. Our designs have been rigorously field tested and can be easily adapted to a variety of form factors.
IRIS is an ideal technology partner for early-stage medical device companies without the budget or expertise to invest in developing a new device from the ground up. Our experienced engineering team and in-house clean-room facilities allow for rapid design development as well as prototype and early-stage commercial production.
IRIS helps companies develop best-in-class products and get to market first. IRIS follows national and international medical device regulations. All design and manufacturing work is performed under an ISO:13485-certified Quality Management System.
The Team Behind IRIS Biomedical

Andrew Wilder, PhD
Chief Executive Officer

Scott Hiatt
Chief Technology Officer
Conferences
We attend a variety of industry events each year
Check the list below for a conference or event near you.
We are attending MD&M West 2023!
February 7-9th, 2023
HEAL Investigators Meeting
February 21-22
Link: https://https://heal.nih.gov/news/events/fourth-heal-investigator-meeting